Three babies have been born after receiving the world’s first spina bifida treatment combining surgery with stem cells. This was made possible by a clinical trial at UC Davis Children’s Hospital in Sacramento, California. The one-of-a-kind treatment, delivered while a fetus is still developing in the mother’s womb, could improve outcomes for children with this congenital disorder.

spina bifida treatment.
Launched in the spring of 2021, the clinical trial is known formally as the “CuRe Trial: Cellular Therapy for In Utero Repair of Myelomeningocele.” Thirty-five patients will be treated during the trial. The three babies from the trial who have been born so far will be monitored by the research team so they can fully assess the procedure’s safety and effectiveness.
Spina bifida, also known as myelomeningocele, occurs when spinal tissue fails to fuse properly during the early stages of pregnancy. The condition can lead to a range of lifelong cognitive, mobility, urinary and bowel disorders. It affects 1,500 to 2,000 children in the U.S. every year, and it is often diagnosed through ultrasound. While surgery performed after birth can help reduce some of the effects, surgery before birth can prevent or lessen the severity of the fetus’s spinal damage, which worsens over the course of pregnancy.
“I’ve been working toward this day for almost 25 years now,” says Diana Farmer, M.D., the world’s first woman fetal surgeon, surgeon-in-chief at UC Davis Children’s Hospital, professor and chair of surgery at UC Davis Health and the principal investigator on the study.
Long path to success
As a leader of the Management of Myelomeningocele Study (MOMS) clinical trial in the early 2000s, Farmer had previously helped to prove that fetal surgery reduced neurological deficits from spina bifida. Many children in that study showed improvement but still required wheelchairs or leg braces.
Farmer recruited bioengineer Aijun Wang to help take that work to the next level. Together, they launched the UC Davis Health Surgical Bioengineering Laboratory to find ways to use stem cells and bioengineering to advance surgical effectiveness and improve outcomes. Farmer also launched the UC Davis Fetal Care and Treatment Center with fetal surgeon Shinjiro Hirose and the UC Davis Children’s Surgery Center several years ago.
Farmer, Wang and their research team have been working on their novel approach using stem cells in fetal surgery for more than 10 years. Over that time, animal modeling has shown it is capable of preventing the paralysis associated with spina bifida. It is believed that the stem cells work to repair and restore damaged spinal tissue, beyond what surgery can accomplish alone.
"I’ve been working toward this day for almost 25 years,"
Preliminary work by Farmer and Wang proved that prenatal surgery combined with human placenta-derived mesenchymal stromal cells, held in place with a biomaterial scaffold to form a “patch,” helped lambs with spina bifida walk without noticeable disability. “When the baby sheep who received stem cells were born, they were able to stand at birth and they were able to run around almost normally. It was amazing,” Wang says.
When the team refined their surgery and stem cell technique for canines, the treatment also improved the mobility of dogs with naturally occurring spina bifida. A pair of English bulldogs named Darla and Spanky were the world’s first dogs to be successfully treated with surgery and stem cells. Spina bifida, a common congenital disorder in this breed, frequently leaves them with little function in their hindquarters. By their post-surgery re-check at 4 months old, Darla and Spanky were able to walk, run and play.
First human trial
When Emily and her husband Harry learned that they would be first-time parents, they never expected any pregnancy complications. But the day that Emily learned her developing child had spina bifida was also the day she first heard about the CuRe trial. For Emily, it was a lifeline that they couldn’t refuse, even though it meant temporarily moving to Sacramento from Austin, Texas, for the fetal surgery and then for weekly follow-up visits during her pregnancy.
Farmer and Wang’s team manufacture clinical-grade stem cells—mesenchymal stem cells—from placental tissue in the UC Davis Health’s CIRM-funded Institute for Regenerative Cures. The cells are known to be among the most promising type of cells in regenerative medicine. The lab is a Good Manufacturing Practice (GMP) Laboratory for safe use in humans. It is there that they made the stem cell patch for Emily’s fetal surgery.
“It’s a four-day process to make the stem cell patch,” says Priya Kumar, the scientist at the Center for Surgical Bioengineering in the Department of Surgery, who leads the team that creates the stem cell patches and delivers them to the operating room. “The time we pull out the cells, the time we seed on the scaffold, and the time we deliver is all critical.”
A first in medical history
During Emily’s historic procedure, a 40-person operating and cell preparation team did the careful dance that they had been long preparing for. After Emily was placed under general anesthetic, a small opening was made in her uterus, and they floated the fetus up to that incision point so they could expose its spine and the spina bifida defect. The surgeons used a microscope to carefully begin the repair.
Then the moment of truth: The stem cell patch was placed directly over the exposed spinal cord of the fetus. The fetal surgeons then closed the incision to allow the tissue to regenerate. “The placement of the stem cell patch went off without a hitch. Mother and fetus did great!” Farmer says. The team declared the first-of-its-kind surgery a success.
Delivery day
On Sept. 20, 2021, at 35 weeks and five days gestation, Robbie was born via C-section at 5.10 pounds and 19 inches. “One of my first fears was that I wouldn’t be able to see her, but they brought her over to me. I got to see her toes wiggle for the first time. It was so reassuring and a little bit out of this world,” Emily says.
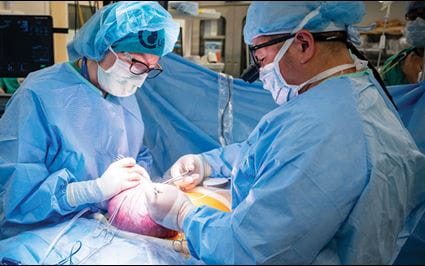
on the fetus’s spinal cord.
For Farmer, this day is what she had long hoped for, and it came with surprises. If Robbie had remained untreated, she was expected to be born with leg paralysis. “It was very clear the minute she was born that she was kicking her legs and I remember very clearly saying, ‘Oh my God, I think she’s wiggling her toes!’” says Farmer. “It was amazing. We kept saying, ‘Am I seeing that? Is that real?’”
Both mom and baby are at home and in good health. Robbie just celebrated her first birthday. The CuRe team is cautious about drawing conclusions and says a lot is still to be learned during this safety phase of the trial. The team will continue to monitor Robbie and the other babies in the trial until they are 6 years old, with a key checkup happening at 30 months to see if they are walking and potty training.
“This experience has been larger than life and has exceeded every expectation,” Emily says. “I hope this trial will enhance the quality of life for so many patients to come. We are honored to be part of history in the making.”


